Abstract
Background & objective: Omidubicel is an investigational advanced cell therapy manufactured from an appropriately HLA-matched single umbilical cord blood (UCB) unit that expands the number of stem cells while preserving stem cell function to optimize cell homing, engraftment, differentiation, and self-renewal. Allogeneic hematopoietic cell transplant (alloHCT) with omidubicel has demonstrated superiority to unmanipulated UCB transplant for patients with hematologic malignancies in a Phase III randomized trial (Horwitz ME, et al. Blood. 2021;138(6):1429-1440). The aim of the current real-world analysis was to compare the effectiveness of omidubicel versus other alloHCT donor sources used in clinical practice from the Center for International Blood and Marrow Transplant Research (CIBMTR) database of all alloHCTs performed in the United States in recent years.
Methods: Data on patients who received omidubicel and unmanipulated UCB were drawn from the per-protocol (treated) population in the Phase III trial (NCT02730299). Data on patients who underwent myeloablative conditioning and first alloHCT for hematologic malignancies between 2017 and 2019 with haploidentical (with post-transplant cyclophosphamide), 8/8-matched unrelated (MUD), or 7/8-matched unrelated (MMUD) donors were obtained from the CIBMTR. Neutrophil engraftment (without chimerism), platelet engraftment, overall survival (OS), disease-free survival (DFS), non-relapse mortality (NRM), disease relapse (DR), and acute and chronic graft-versus-host disease (GvHD) were compared between groups with and without adjustment for baseline demographics and disease factors (age, gender, race, ethnicity, primary diagnosis, disease risk index, performance score, HCT comorbidity index, body weight, year of transplant and conditioning regimen). Analyses utilized Kaplan-Meier analyses, Cox proportional hazards models, and Fine-Gray models for outcomes with competing risks.
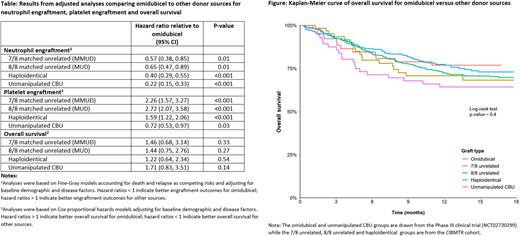
Results: Fifty-two patients received omidubicel, 56 received unmanipulated UCB, and 807 patients received other donor sources. Omidubicel was associated with more rapid neutrophil recovery (median: 10 days) than all other donor sources (median 15-20 days; log-rank p-value <0.001). Omidubicel was associated with slower platelet engraftment than the haploidentical, MUD or MMUD donor sources and faster platelet engraftment than unmanipulated UCB (median: 35 days versus 19-26 days for haploidentical, MUD and MMUD, and 43 days for unmanipulated UCB; log-rank p-value <0.001). Results were similar in analyses accounting for death and relapse as competing risks and robust to adjustment for baseline demographic and disease factors (Table). OS was comparable across donor sources in unadjusted (Figure) and adjusted analyses (Table). DFS, NRM and DR were also comparable across donor sources.
Rates of grade II-IV acute GvHD over the first 100 days post alloHCT were significantly higher for omidubicel (60% versus 32-43% for other sources, p-values from adjusted analyses <0.05 for all sources relative to omidubicel). However, rates of grade III-IV acute GvHD over the same time frame (15% for omidubicel versus 10-20% for other sources) and chronic GvHD over 12 months post alloHCT (33% for omidubicel versus 25-42% for other sources) were comparable across donor sources (p-values from adjusted analyses >0.05 for all sources relative to omidubicel).
Conclusion: In this adjusted comparison to external CIBMTR controls receiving haploidentical or MUD/ MMUD donor sources, omidubicel was associated with earlier neutrophil engraftment, comparable OS, and comparable rates of acute GVHD grades III-IV and chronic GvHD. While this study used high-quality real-world data, harmonized inclusion criteria and outcome definitions, and adjustment for prognostic factors to mitigate risk of bias, the comparison of non-randomized treatment groups should be interpreted with caution. These results suggest that omidubicel may be an effective and important additional donor source, broadening the availability of transplantation for patients with hematologic malignancies.
Disclosures
Sivaraman:Gamida Cell, Inc.: Current Employment, Current equity holder in publicly-traded company; Incyte Corporation: Ended employment in the past 24 months. Sajeev:Gamida Cell, Inc.: Consultancy. Song:Gamida Cell, Inc.: Consultancy; Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Xu:Gamida Cell, Inc.: Consultancy. He:Gamida Cell, Inc.: Consultancy. Signorovitch:Gamida Cell, Inc.: Consultancy; Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Shin:Gamida Cell, Inc.: Current Employment; Bristol Myers Squibb: Ended employment in the past 24 months. Manghani:Gamida Cell, Inc.: Current Employment, Current equity holder in publicly-traded company. Tang:Bristol Myers Squibb: Research Funding; Kite Pharma: Other: Spouse is currently employed at Kite; Gamida-Cell Ltd: Research Funding. Burns:Astellas Pharma Inc.: Research Funding; Bristol Myers Squibb: Research Funding; Gamida Cell: Research Funding. Simantov:Gamida Cell, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal